Petroleum (Crude Oil)
Petroleum
|
|
This article needs to be updated. (October 2017)
|
Pumpjack pumping an oil well near Lubbock, Texas
An oil refinery in Mina Al Ahmadi, Kuwait
It consists of hydrocarbons of various molecular weights and other organic compounds.[1][2] The name petroleum covers both naturally occurring unprocessed crude oil and petroleum products that are made up of refined crude oil. A fossil fuel, petroleum is formed when large quantities of dead organisms, usually zooplankton and algae, are buried underneath sedimentary rock and subjected to both intense heat and pressure.
Petroleum has mostly been recovered by oil drilling (natural petroleum springs are rare). Drilling is carried out after studies of structural geology (at the reservoir scale), sedimentary basin analysis, and reservoir characterisation (mainly in terms of the porosity and permeability of geologic reservoir structures) have been completed.[3][4] It is refined and separated, most easily by distillation, into a large number of consumer products, from gasoline (petrol) and kerosene to asphalt and chemical reagents used to make plastics and pharmaceuticals.[5] Petroleum is used in manufacturing a wide variety of materials,[6] and it is estimated that the world consumes about 95 million barrels each day.
Concern over the depletion of the earth's finite reserves of oil, and the effect this would have on a society dependent on it, is a concept known as peak oil. The use of fossil fuels, such as petroleum, has a negative impact on Earth's biosphere, damaging ecosystems through events such as oil spills and releasing a range of pollutants into the air including ground-level ozone and sulfur dioxide from sulfur impurities in fossil fuels. The burning of fossil fuels plays a major role in the current episode of global warming.
Contents
- 1 Etymology
- 2 History
- 3 Composition
- 4 Chemistry
- 5 Empirical equations for thermal properties
- 6 Formation
- 7 Reservoirs
- 8 Classification
- 9 Petroleum industry
- 10 Price
- 11 Uses
- 12 Petroleum by country
- 13 Environmental effects
- 14 Alternatives to petroleum
- 15 Future of petroleum production
- 16 See also
- 17 Notes
- 18 References
- 19 Further reading
- 20 External links
Etymology
Fractional distillation apparatus
The term was found (in the spelling "petraoleum") in 10th-century Old English sources.[12][not in citation given] It was used in the treatise De Natura Fossilium, published in 1546 by the German mineralogist Georg Bauer, also known as Georgius Agricola.[13] In the 19th century, the term petroleum was often used to refer to mineral oils produced by distillation from mined organic solids such as cannel coal (and later oil shale), and refined oils produced from them; in the United Kingdom, storage (and later transport) of these oils were regulated by a series of Petroleum Acts, from the Petroleum Act 1863 onwards.
History
Early history
Oil derrick in Okemah, Oklahoma, 1922
More than 4000 years ago, according to Herodotus and Diodorus Siculus, asphalt was used in the construction of the walls and towers of Babylon; there were oil pits near Ardericca (near Babylon), and a pitch spring on Zacynthus.[14] Great quantities of it were found on the banks of the river Issus, one of the tributaries of the Euphrates. Ancient Persian tablets indicate the medicinal and lighting uses of petroleum in the upper levels of their society.
The use of petroleum dates back to ancient China more than 2000 years ago. In I Ching, one of the earliest Chinese writings cites the use of oil in its raw state without refining was first discovered, extracted, and used in China in the first century BCE. In addition, the Chinese were the first to use petroleum as fuel as the early as the fourth century BCE.[15][16][17]
By 347 AD, oil was produced from bamboo-drilled wells in China.[18][19] Early British explorers to Myanmar documented a flourishing oil extraction industry based in Yenangyaung that, in 1795, had hundreds of hand-dug wells under production.[20]
Pechelbronn (Pitch fountain) is said to be the first European site where petroleum has been explored and used. The still active Erdpechquelle, a spring where petroleum appears mixed with water has been used since 1498, notably for medical purposes. Oil sands have been mined since the 18th century.[21]
In Wietze in lower Saxony, natural asphalt/bitumen has been explored since the 18th century.[22] Both in Pechelbronn as in Wietze, the coal industry dominated the petroleum technologies.[23]
Modern history
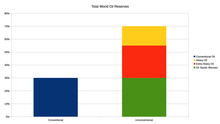
Proven world oil reserves, 2013. Unconventional reservoirs such as natural heavy oil and oil sands are included.
Young eventually succeeded, by distilling cannel coal at a low heat, in creating a fluid resembling petroleum, which when treated in the same way as the seep oil gave similar products. Young found that by slow distillation he could obtain a number of useful liquids from it, one of which he named "paraffine oil" because at low temperatures it congealed into a substance resembling paraffin wax.[24]
The production of these oils and solid paraffin wax from coal formed the subject of his patent dated 17 October 1850. In 1850 Young & Meldrum and Edward William Binney entered into partnership under the title of E.W. Binney & Co. at Bathgate in West Lothian and E. Meldrum & Co. at Glasgow; their works at Bathgate were completed in 1851 and became the first truly commercial oil-works in the world with the first modern oil refinery,[25] using oil extracted from locally mined torbanite, shale, and bituminous coal to manufacture naphtha and lubricating oils; paraffin for fuel use and solid paraffin were not sold until 1856.[citation needed]
Shale bings near Broxburn, 3 of a total of 19 in West Lothian
The demand for petroleum as a fuel for lighting in North America and around the world quickly grew.[28] Edwin Drake's 1859 well near Titusville, Pennsylvania, is popularly considered the first modern well. Already 1858 Georg Christian Konrad Hunäus had found a significant amount of petroleum while drilling for lignite 1858 in Wietze, Germany. Wietze later provided about 80% of the German consumption in the Wilhelminian Era.[29] The production stopped in 1963, but Wietze has hosted a Petroleum Museum since 1970.[30]
Drake's well is probably singled out because it was drilled, not dug; because it used a steam engine; because there was a company associated with it; and because it touched off a major boom.[31] However, there was considerable activity before Drake in various parts of the world in the mid-19th century. A group directed by Major Alexeyev of the Bakinskii Corps of Mining Engineers hand-drilled a well in the Baku region in 1848.[32] There were engine-drilled wells in West Virginia in the same year as Drake's well.[33] An early commercial well was hand dug in Poland in 1853, and another in nearby Romania in 1857. At around the same time the world's first, small, oil refinery was opened at Jasło in Poland, with a larger one opened at Ploiești in Romania shortly after. Romania is the first country in the world to have had its annual crude oil output officially recorded in international statistics: 275 tonnes for 1857.[34][35]
The first commercial oil well in Canada became operational in 1858 at Oil Springs, Ontario (then Canada West).[36] Businessman James Miller Williams dug several wells between 1855 and 1858 before discovering a rich reserve of oil four metres below ground.[37][specify] Williams extracted 1.5 million litres of crude oil by 1860, refining much of it into kerosene lamp oil. Williams's well became commercially viable a year before Drake's Pennsylvania operation and could be argued to be the first commercial oil well in North America.[38] The discovery at Oil Springs touched off an oil boom which brought hundreds of speculators and workers to the area. Advances in drilling continued into 1862 when local driller Shaw reached a depth of 62 metres using the spring-pole drilling method.[39] On January 16, 1862, after an explosion of natural gas Canada's first oil gusher came into production, shooting into the air at a recorded rate of 3,000 barrels per day.[40] By the end of the 19th century the Russian Empire, particularly the Branobel company in Azerbaijan, had taken the lead in production.[41]
Access to oil was and still is a major factor in several military conflicts of the twentieth century, including World War II, during which oil facilities were a major strategic asset and were extensively bombed.[42] The German invasion of the Soviet Union included the goal to capture the Baku oilfields, as it would provide much needed oil-supplies for the German military which was suffering from blockades.[43] Oil exploration in North America during the early 20th century later led to the US becoming the leading producer by mid-century. As petroleum production in the US peaked during the 1960s, however, the United States was surpassed by Saudi Arabia and the Soviet Union.[citation needed]
Today, about 90 percent of vehicular fuel needs are met by oil. Petroleum also makes up 40 percent of total energy consumption in the United States, but is responsible for only 1 percent of electricity generation.[44] Petroleum's worth as a portable, dense energy source powering the vast majority of vehicles and as the base of many industrial chemicals makes it one of the world's most important commodities. Viability of the oil commodity is controlled by several key parameters, number of vehicles in the world competing for fuel, quantity of oil exported to the world market (Export Land Model), net energy gain (economically useful energy provided minus energy consumed), political stability of oil exporting nations and ability to defend oil supply lines.ci[citation needed]
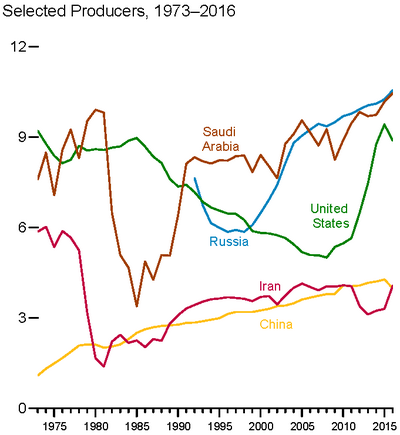
The top three oil producing countries are Russia, Saudi Arabia and the United States.[45] About 80 percent of the world's readily accessible reserves are located in the Middle East, with 62.5 percent coming from the Arab 5: Saudi Arabia, United Arab Emirates, Iraq, Qatar and Kuwait. A large portion of the world's total oil exists as unconventional sources, such as bitumen in Athabasca oil sands and extra heavy oil in the Orinoco Belt. While significant volumes of oil are extracted from oil sands, particularly in Canada, logistical and technical hurdles remain, as oil extraction requires large amounts of heat and water, making its net energy content quite low relative to conventional crude oil. Thus, Canada's oil sands are not expected to provide more than a few million barrels per day in the foreseeable future.[citation needed]
Composition
In its strictest sense, petroleum includes only crude oil, but in common usage it includes all liquid, gaseous and solid hydrocarbons. Under surface pressure and temperature conditions, lighter hydrocarbons methane, ethane, propane and butane occur as gases, while pentane and heavier hydrocarbons are in the form of liquids or solids. However, in an underground oil reservoir the proportions of gas, liquid, and solid depend on subsurface conditions and on the phase diagram of the petroleum mixture.[46]An oil well produces predominantly crude oil, with some natural gas dissolved in it. Because the pressure is lower at the surface than underground, some of the gas will come out of solution and be recovered (or burned) as associated gas or solution gas. A gas well produces predominantly natural gas. However, because the underground temperature and pressure are higher than at the surface, the gas may contain heavier hydrocarbons such as pentane, hexane, and heptane in the gaseous state. At surface conditions these will condense out of the gas to form "natural gas condensate", often shortened to condensate. Condensate resembles gasoline in appearance and is similar in composition to some volatile light crude oils.[citation needed]
The proportion of light hydrocarbons in the petroleum mixture varies greatly among different oil fields, ranging from as much as 97 percent by weight in the lighter oils to as little as 50 percent in the heavier oils and bitumens.[citation needed]
The hydrocarbons in crude oil are mostly alkanes, cycloalkanes and various aromatic hydrocarbons, while the other organic compounds contain nitrogen, oxygen and sulfur, and trace amounts of metals such as iron, nickel, copper and vanadium. Many oil reservoirs contain live bacteria.[47] The exact molecular composition of crude oil varies widely from formation to formation but the proportion of chemical elements varies over fairly narrow limits as follows:[48]
| Element | Percent range |
|---|---|
| Carbon | 83 to 85% |
| Hydrogen | 10 to 14% |
| Nitrogen | 0.1 to 2% |
| Oxygen | 0.05 to 1.5% |
| Sulfur | 0.05 to 6.0% |
| Metals | < 0.1% |
| Hydrocarbon | Average | Range |
|---|---|---|
| Alkanes (paraffins) | 30% | 15 to 60% |
| Naphthenes | 49% | 30 to 60% |
| Aromatics | 15% | 3 to 30% |
| Asphaltics | 6% | remainder |
Unconventional resources are much larger than conventional ones.[49]
Petroleum is used mostly, by volume, for producing fuel oil and gasoline, both important "primary energy" sources. 84 percent by volume of the hydrocarbons present in petroleum is converted into energy-rich fuels (petroleum-based fuels), including gasoline, diesel, jet, heating, and other fuel oils, and liquefied petroleum gas.[52] The lighter grades of crude oil produce the best yields of these products, but as the world's reserves of light and medium oil are depleted, oil refineries are increasingly having to process heavy oil and bitumen, and use more complex and expensive methods to produce the products required. Because heavier crude oils have too much carbon and not enough hydrogen, these processes generally involve removing carbon from or adding hydrogen to the molecules, and using fluid catalytic cracking to convert the longer, more complex molecules in the oil to the shorter, simpler ones in the fuels.[citation needed]
Due to its high energy density, easy transportability and relative abundance, oil has become the world's most important source of energy since the mid-1950s. Petroleum is also the raw material for many chemical products, including pharmaceuticals, solvents, fertilizers, pesticides, and plastics; the 16 percent not used for energy production is converted into these other materials. Petroleum is found in porous rock formations in the upper strata of some areas of the Earth's crust. There is also petroleum in oil sands (tar sands). Known oil reserves are typically estimated at around 190 km3 (1.2 trillion (short scale) barrels) without oil sands,[53] or 595 km3 (3.74 trillion barrels) with oil sands.[54] Consumption is currently around 84 million barrels (13.4×106 m3) per day, or 4.9 km3 per year, yielding a remaining oil supply of only about 120 years, if current demand remains static.[citation needed]
Chemistry
Octane, a hydrocarbon found in petroleum. Lines represent single bonds; black spheres represent carbon; white spheres represent hydrogen.
The alkanes, also known as paraffins, are saturated hydrocarbons with straight or branched chains which contain only carbon and hydrogen and have the general formula CnH2n+2. They generally have from 5 to 40 carbon atoms per molecule, although trace amounts of shorter or longer molecules may be present in the mixture.
The alkanes from pentane (C5H12) to octane (C8H18) are refined into gasoline, the ones from nonane (C9H20) to hexadecane (C16H34) into diesel fuel, kerosene and jet fuel. Alkanes with more than 16 carbon atoms can be refined into fuel oil and lubricating oil. At the heavier end of the range, paraffin wax is an alkane with approximately 25 carbon atoms, while asphalt has 35 and up, although these are usually cracked by modern refineries into more valuable products. The shortest molecules, those with four or fewer carbon atoms, are in a gaseous state at room temperature. They are the petroleum gases. Depending on demand and the cost of recovery, these gases are either flared off, sold as liquefied petroleum gas under pressure, or used to power the refinery's own burners. During the winter, butane (C4H10), is blended into the gasoline pool at high rates, because its high vapor pressure assists with cold starts. Liquified under pressure slightly above atmospheric, it is best known for powering cigarette lighters, but it is also a main fuel source for many developing countries. Propane can be liquified under modest pressure, and is consumed for just about every application relying on petroleum for energy, from cooking to heating to transportation.
The cycloalkanes, also known as naphthenes, are saturated hydrocarbons which have one or more carbon rings to which hydrogen atoms are attached according to the formula CnH2n. Cycloalkanes have similar properties to alkanes but have higher boiling points.
The aromatic hydrocarbons are unsaturated hydrocarbons which have one or more planar six-carbon rings called benzene rings, to which hydrogen atoms are attached with the formula CnH2n-6. They tend to burn with a sooty flame, and many have a sweet aroma. Some are carcinogenic.
These different molecules are separated by fractional distillation at an oil refinery to produce gasoline, jet fuel, kerosene, and other hydrocarbons. For example, 2,2,4-trimethylpentane (isooctane), widely used in gasoline, has a chemical formula of C8H18 and it reacts with oxygen exothermically:[55]
- 2 C
8H
18(l) + 25 O
2(g) → 16 CO
2(g) + 18 H
2O(g) (ΔH = −5.51 MJ/mol of octane)
Incomplete combustion of petroleum or gasoline results in production of toxic byproducts. Too little oxygen during combustion results in the formation of carbon monoxide. Due to the high temperatures and high pressures involved, exhaust gases from gasoline combustion in car engines usually include nitrogen oxides which are responsible for creation of photochemical smog.
Empirical equations for thermal properties
Heat of combustion
At a constant volume, the heat of combustion of a petroleum product can be approximated as follows:,
 is measured in calories per gram and
is measured in calories per gram and  is the specific gravity at 60 °F (16 °C).
is the specific gravity at 60 °F (16 °C).Thermal conductivity
The thermal conductivity of petroleum based liquids can be modeled as follows:[57] is measured in BTU · °F−1hr−1ft−1 ,
is measured in BTU · °F−1hr−1ft−1 ,  is measured in °F and
is measured in °F and  is degrees API gravity.
is degrees API gravity.Specific heat
The specific heat of petroleum oils can be modeled as follows:[58],
 is measured in BTU/(lb °F),
is measured in BTU/(lb °F),  is the temperature in Fahrenheit and
is the temperature in Fahrenheit and  is the specific gravity at 60 °F (16 °C).
is the specific gravity at 60 °F (16 °C).In units of kcal/(kg·°C), the formula is:
,
 is in Celsius and
is in Celsius and  is the specific gravity at 15 °C.
is the specific gravity at 15 °C.Latent heat of vaporization
The latent heat of vaporization can be modeled under atmospheric conditions as follows:,
 is measured in BTU/lb,
is measured in BTU/lb,  is measured in °F and
is measured in °F and  is the specific gravity at 60 °F (16 °C).
is the specific gravity at 60 °F (16 °C).In units of kcal/kg, the formula is:
,
 is in Celsius and
is in Celsius and  is the specific gravity at 15 °C.[59]
is the specific gravity at 15 °C.[59]Formation
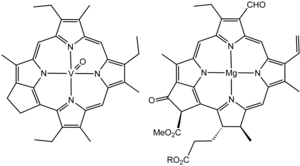
Structure of a vanadium porphyrin compound (left) extracted from petroleum by Alfred E. Treibs, father of organic geochemistry. Treibs noted the close structural similarity of this molecule and chlorophyll a (right).[60][61]
As further layers settled to the sea or lake bed, intense heat and pressure built up in the lower regions. This process caused the organic matter to change, first into a waxy material known as kerogen, found in various oil shales around the world, and then with more heat into liquid and gaseous hydrocarbons via a process known as catagenesis. Formation of petroleum occurs from hydrocarbon pyrolysis in a variety of mainly endothermic reactions at high temperature or pressure, or both.[2][63] These phases are decribed in detail below.
Anaerobic decay or 1. phase of diagenesis
In the absence of plentiful oxygen, aerobic bacteria were prevented from decaying the organic matter. Anaerobic bacteria were able to reduce sulfates and nitrates to H2S and N2 respectively by using the organic matter as a source for reactants. Due to such bacteria, this matter began to break mostly trough hydrolysis at first: polysaccharides and proteins were hydrolyzed to simple sugars and amino acids respectively. These were further anaerobically oxidized at an accelerated rate by the enzymes of the bacteria: e.g. amino acids went through oxidative deamination to imino acids, which in turn reacted futher to ammonia and α-keto acids. Monosaccharides in turn ultimately decayed to CO2 and methane. The products of amino acids, monosaccharides, phenols and aldehydes combined to fulvic acids. Fats and waxes were not extensively hydrolyzed under these mild conditions.[2]Kerogen formation or 2. phase of diagenesis
Some phenolic compounds produced from previous reactions worked as bactericides and actinomycetales order of bacteria produced antibiotic compounds (e.g. streptomycin). Thus the action of anaerobic bacteria ceased about 10 m below the water or sediment. The mixture at this depth contained fulvic acids, unreacted and partially reacted fats and waxes, slighty modified lignin, resins and other hydrocarbons.[2] As further layers settled to the sea or lake bed, intense heat and pressure built up in the lower regions.[63] As a consequence, compounds of this mixture the began to combine in poorly understood ways to kerogen. Combination happened in a similar fashion as phenol and formaldehyde molecules react to urea-formaldehyde resins, but happened in a more complex manner due to bigger variety of reactants. The total process of kerogen formation from the beginning of anaerobic decay is called diagenesis, a word that means a transformation of materials by dissolution and recombination of their constituents.[2]Kerogen to fossil fuels or catagenesis
Kerogen formation continues to the depth of about 1000 m where temperatures may reach around 50 °C. Kerogen formation represents a halfway point between organic matter and fossil fuels: kerogen can be exposed to oxygen, oxidize and be lost or it could be buried increasingly deeper inside the Earth's crust and to conditions which allow it to slowly transform into fossil fuels. The latter happens trough catagenesis in which the reactions are mostly radical rearrangements of kerogen, take thousands to millions of years and no external reactants are involved. Due to radical nature of the reactions, kerogen reacts towards two classes of products: those with high hydrogen/carbon ratio (anthracene or similar to it) and those with high H/C ratio (methane or similar to it). Because catagenesis is closed off from external reactants, the resulting composition of the fuel mixture formed is dependent on the composition (stoichiometry) of the kerogen. 3 main types of kerogen exist: type I (algal), II (liptinic) and III (humic), which are formed mainly from algae, plankton and woody plants (this term includes trees, shrubs and lianas) respectively.[2]Catagenesis is pyrolytic despite of the fact that it happens at low temperatures of 60 to several hundred °C. Pyrolysis is possible because of the long reaction times involved. Heat for catagenesis comes from the decomposition of radioactive materials of the crust, especially 40K, 232Th, 235U and 238U. The heat varies with geothermal gradient and is typically 10-30 °C per km. Unusual magma intrusions can create greater localised heating.[2]
Geologists often refer to the temperature range in which oil forms as an "oil window"[64][2] - below the minimum temperature oil remains trapped in the form of kerogen. Above the maximum temperature the oil is converted to natural gas through the process of thermal cracking. Sometimes, oil formed at extreme depths may migrate and become trapped at a much shallower level. The Athabasca Oil Sands are one example of this.[2]
Abiogenic petroleum
An alternative mechanism was proposed by Russian scientists in the mid-1850s, the hypothesis of abiogenic petroleum origin (petroleum formed by inorganic means), but this is contradicted by geological and geochemical evidence.[65] Abiogenic sources of oil have been found, but never in commercially profitable amounts. "The controversy isn't over whether abiogenic oil reserves exist," said Larry Nation of the American Association of Petroleum Geologists. "The controversy is over how much they contribute to Earth's overall reserves and how much time and effort geologists should devote to seeking them out."[66]Reservoirs
|
This section needs additional citations for verification. (October 2016) (Learn how and when to remove this template message)
|
Hydrocarbon trap
- a source rock rich in hydrocarbon material buried deeply enough for subterranean heat to cook it into oil,
- a porous and permeable reservoir rock where it can accumulate,
- a caprock (seal) or other mechanism to prevent the oil from escaping to the surface. Within these reservoirs, fluids will typically organize themselves like a three-layer cake with a layer of water below the oil layer and a layer of gas above it, although the different layers vary in size between reservoirs. Because most hydrocarbons are less dense than rock or water, they often migrate upward through adjacent rock layers until either reaching the surface or becoming trapped within porous rocks (known as reservoirs) by impermeable rocks above. However, the process is influenced by underground water flows, causing oil to migrate hundreds of kilometres horizontally or even short distances downward before becoming trapped in a reservoir. When hydrocarbons are concentrated in a trap, an oil field forms, from which the liquid can be extracted by drilling and pumping.
Wells are drilled into oil reservoirs to extract the crude oil. "Natural lift" production methods that rely on the natural reservoir pressure to force the oil to the surface are usually sufficient for a while after reservoirs are first tapped. In some reservoirs, such as in the Middle East, the natural pressure is sufficient over a long time. The natural pressure in most reservoirs, however, eventually dissipates. Then the oil must be extracted using "artificial lift" means. Over time, these "primary" methods become less effective and "secondary" production methods may be used. A common secondary method is "waterflood" or injection of water into the reservoir to increase pressure and force the oil to the drilled shaft or "wellbore." Eventually "tertiary" or "enhanced" oil recovery methods may be used to increase the oil's flow characteristics by injecting steam, carbon dioxide and other gases or chemicals into the reservoir. In the United States, primary production methods account for less than 40 percent of the oil produced on a daily basis, secondary methods account for about half, and tertiary recovery the remaining 10 percent. Extracting oil (or "bitumen") from oil/tar sand and oil shale deposits requires mining the sand or shale and heating it in a vessel or retort, or using "in-situ" methods of injecting heated liquids into the deposit and then pumping the liquid back out saturated with oil.
Unconventional oil reservoirs
Oil-eating bacteria biodegrade oil that has escaped to the surface. Oil sands are reservoirs of partially biodegraded oil still in the process of escaping and being biodegraded, but they contain so much migrating oil that, although most of it has escaped, vast amounts are still present—more than can be found in conventional oil reservoirs. The lighter fractions of the crude oil are destroyed first, resulting in reservoirs containing an extremely heavy form of crude oil, called crude bitumen in Canada, or extra-heavy crude oil in Venezuela. These two countries have the world's largest deposits of oil sands.[citation needed]On the other hand, oil shales are source rocks that have not been exposed to heat or pressure long enough to convert their trapped hydrocarbons into crude oil. Technically speaking, oil shales are not always shales and do not contain oil, but are fined-grain sedimentary rocks containing an insoluble organic solid called kerogen. The kerogen in the rock can be converted into crude oil using heat and pressure to simulate natural processes. The method has been known for centuries and was patented in 1694 under British Crown Patent No. 330 covering, "A way to extract and make great quantities of pitch, tar, and oil out of a sort of stone." Although oil shales are found in many countries, the United States has the world's largest deposits.[67]
Classification
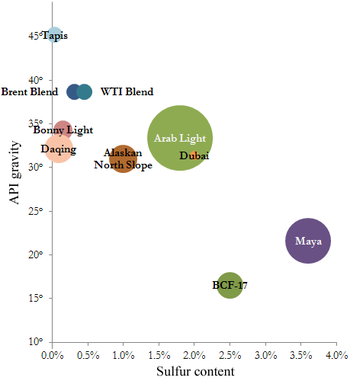
Some marker crudes with their sulfur content (horizontal) and API gravity (vertical) and relative production quantity
The geographic location is important because it affects transportation costs to the refinery. Light crude oil is more desirable than heavy oil since it produces a higher yield of gasoline, while sweet oil commands a higher price than sour oil because it has fewer environmental problems and requires less refining to meet sulfur standards imposed on fuels in consuming countries. Each crude oil has unique molecular characteristics which are revealed by the use of Crude oil assay analysis in petroleum laboratories.[citation needed]
Barrels from an area in which the crude oil's molecular characteristics have been determined and the oil has been classified are used as pricing references throughout the world. Some of the common reference crudes are:[citation needed]
- West Texas Intermediate (WTI), a very high-quality, sweet, light oil delivered at Cushing, Oklahoma for North American oil
- Brent Blend, consisting of 15 oils from fields in the Brent and Ninian systems in the East Shetland Basin of the North Sea. The oil is landed at Sullom Voe terminal in Shetland. Oil production from Europe, Africa and Middle Eastern oil flowing West tends to be priced off this oil, which forms a benchmark
- Dubai-Oman, used as benchmark for Middle East sour crude oil flowing to the Asia-Pacific region
- Tapis (from Malaysia, used as a reference for light Far East oil)
- Minas (from Indonesia, used as a reference for heavy Far East oil)
- The OPEC Reference Basket, a weighted average of oil blends from various OPEC (The Organization of the Petroleum Exporting Countries) countries
- Midway Sunset Heavy, by which heavy oil in California is priced[68][not in citations given]
- Western Canadian Select the benchmark crude oil for emerging heavy, high TAN (acidic) crudes.[69]
Petroleum industry
|
|
This article needs to be updated. (April 2016)
|
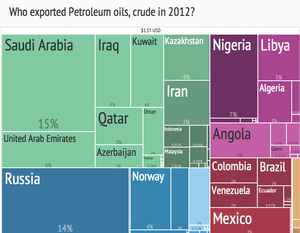
Crude oil export treemap (2012) from Harvard Atlas of Economic Complexity[71]
New York Mercantile Exchange prices ($/bbl) for West Texas Intermediate 2000 through Oct 2014
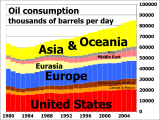
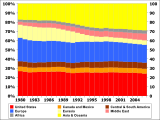
Petroleum is vital to many industries, and is of importance to the maintenance of industrialized civilization itself, and thus is a critical concern to many nations. Oil accounts for a large percentage of the world's energy consumption, ranging from a low of 32 percent for Europe and Asia, up to a high of 53 percent for the Middle East, South and Central America (44%), Africa (41%), and North America (40%). The world at large consumes 30 billion barrels (4.8 km³) of oil per year, and the top oil consumers largely consist of developed nations. In fact, 24 percent of the oil consumed in 2004 went to the United States alone,[72] though by 2007 this had dropped to 21 percent of world oil consumed.[73]
In the US, in the states of Arizona, California, Hawaii, Nevada, Oregon and Washington, the Western States Petroleum Association (WSPA) represents companies responsible for producing, distributing, refining, transporting and marketing petroleum. This non-profit trade association was founded in 1907, and is the oldest petroleum trade association in the United States.[74]
Shipping
In the 1950s, shipping costs made up 33 percent of the price of oil transported from the Persian Gulf to USA,[75] but due to the development of supertankers in the 1970s, the cost of shipping dropped to only 5 percent of the price of Persian oil in USA.[75] Due to the increase of the value of the crude oil during the last 30 years, the share of the shipping cost on the final cost of the delivered commodity was less than 3% in 2010. For example, in 2010 the shipping cost from the Persian Gulf to the USA was in the range of 20 $/t and the cost of the delivered crude oil around 800 $/t.[citation needed]Price
Nominal and inflation-adjusted US dollar price of crude oil, 1861–2015
Uses
The chemical structure of petroleum is heterogeneous, composed of hydrocarbon chains of different lengths. Because of this, petroleum may be taken to oil refineries and the hydrocarbon chemicals separated by distillation and treated by other chemical processes, to be used for a variety of purposes. The total cost of a plant is about 9 billion dollars per plant.Fuels
A poster used to promote carpooling as a way to ration gasoline during World War II
| Fraction | Boiling range oC |
|---|---|
| Liquefied petroleum gas (LPG) | −40 |
| Butane | −12 to −1 |
| Gasoline | −1 to 110 |
| Jet fuel | 150 to 205 |
| Kerosene | 205 to 260 |
| Fuel oil | 205 to 290 |
| Diesel fuel | 260 to 315 |
| Class of petroleum | Composition of 250–300 °C fraction, wt. % |
||||
|---|---|---|---|---|---|
| Par. | Napth | Arom. | Wax | Asph. | |
| Paraffinic | 46—61 | 22–32 | 12–25 | 1.5–10 | 0–6 |
| Paraffinic-naphtenic | 42–45 | 38–39 | 16–20 | 1–6 | 0–6 |
| Naphthenic | 15–26 | 61–76 | 8–13 | Trace | 0–6 |
| Paraffinic-naphtenic-aromatic | 27–35 | 36–47 | 26–33 | 0.5–1 | 0–10 |
| Aromatic | 0–8 | 57–78 | 20–25 | 0–0.5 | 0–20 |
Other derivatives
Certain types of resultant hydrocarbons may be mixed with other non-hydrocarbons, to create other end products:[citation needed]- Alkenes (olefins), which can be manufactured into plastics or other compounds
- Lubricants (produces light machine oils, motor oils, and greases, adding viscosity stabilizers as required)
- Wax, used in the packaging of frozen foods, among others
- Sulfur or sulfuric acid. These are useful industrial materials. Sulfuric acid is usually prepared as the acid precursor oleum, a byproduct of sulfur removal from fuels.
- Bulk tar
- Asphalt
- Petroleum coke, used in speciality carbon products or as solid fuel
- Paraffin wax
- Aromatic petrochemicals to be used as precursors in other chemical production
Agriculture
Since the 1940s, agricultural productivity has increased dramatically, due largely to the increased use of energy-intensive mechanization, fertilizers and pesticides.Petroleum by country
Consumption statistics
-
Rate of world energy usage per year from 1970.[78]
Consumption
According to the US Energy Information Administration (EIA) estimate for 2011, the world consumes 87.421 million barrels of oil each day.
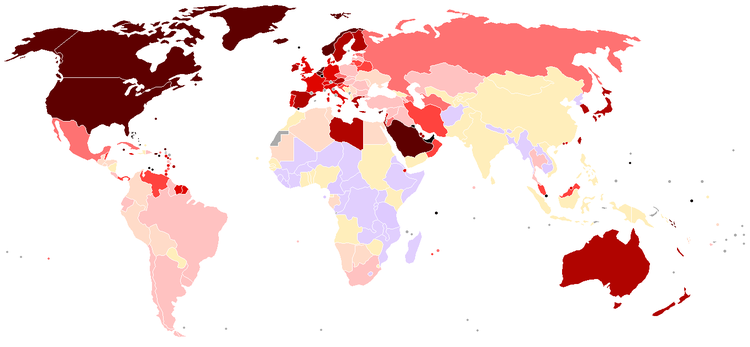
Oil consumption per capita (darker colors represent more consumption, gray represents no data) (source: see file description)
| > 0.07 0.07 - 0.05 0.05 - 0.035 0.035 - 0.025 0.025 - 0.02 |
0.02 - 0.015 0.015 - 0.01 0.01 - 0.005 0.005 - 0.0015 < 0.0015 |
| Consuming nation 2011 | (1000 bbl/ day) |
(1000 m3/ day) |
Population in millions |
bbl/year per capita |
m3/year per capita |
National production/ consumption |
|---|---|---|---|---|---|---|
| United States 1 | 18,835.5 | 2,994.6 | 314 | 21.8 | 3.47 | 0.51 |
| China | 9,790.0 | 1,556.5 | 1345 | 2.7 | 0.43 | 0.41 |
| Japan 2 | 4,464.1 | 709.7 | 127 | 12.8 | 2.04 | 0.03 |
| India 2 | 3,292.2 | 523.4 | 1198 | 1 | 0.16 | 0.26 |
| Russia 1 | 3,145.1 | 500.0 | 140 | 8.1 | 1.29 | 3.35 |
| Saudi Arabia (OPEC) | 2,817.5 | 447.9 | 27 | 40 | 6.4 | 3.64 |
| Brazil | 2,594.2 | 412.4 | 193 | 4.9 | 0.78 | 0.99 |
| Germany 2 | 2,400.1 | 381.6 | 82 | 10.7 | 1.70 | 0.06 |
| Canada | 2,259.1 | 359.2 | 33 | 24.6 | 3.91 | 1.54 |
| South Korea 2 | 2,230.2 | 354.6 | 48 | 16.8 | 2.67 | 0.02 |
| Mexico 1 | 2,132.7 | 339.1 | 109 | 7.1 | 1.13 | 1.39 |
| France 2 | 1,791.5 | 284.8 | 62 | 10.5 | 1.67 | 0.03 |
| Iran (OPEC) | 1,694.4 | 269.4 | 74 | 8.3 | 1.32 | 2.54 |
| United Kingdom 1 | 1,607.9 | 255.6 | 61 | 9.5 | 1.51 | 0.93 |
| Italy 2 | 1,453.6 | 231.1 | 60 | 8.9 | 1.41 | 0.10 |
Population Data:[81]
1 peak production of oil already passed in this state
2 This country is not a major oil producer
Production
Top oil-producing countries (million barrels per day)
World map with countries by oil production (information from 2006–2012)
| Country | Oil Production (bbl/day, 2016)[82] |
|
|---|---|---|
| 1 | 10,551,497 | |
| 2 | 10,460,710 | |
| 3 | 8,875,817 | |
| 4 | 4,451,516 | |
| 5 | 3,990,956 | |
| 6 | 3,980,650 | |
| 7 | 3,662,694 | |
| 8 | 3,106,077 | |
| 9 | 2,923,825 | |
| 10 | 2,515,459 | |
| 11 | 2,276,967 | |
| 12 | 2,186,877 | |
| 13 | 1,999,885 | |
| 14 | 1,769,615 | |
| 15 | 1,647,975 | |
| 16 | 1,595,199 | |
| 17 | 1,522,902 | |
| 18 | 1,348,361 | |
| 19 | 1,006,841 | |
| 20 | 939,760 |
Export
Petroleum Exports by Country (2014) from Harvard Atlas of Economic Complexity
Oil exports by country (barrels per day, 2006)
| # | Exporting nation | 103bbl/d (2011) | 103m3/d (2011) | 103bbl/d (2009) | 103m3/d (2009) | 103bbl/d (2006) | 103m3/d (2006) |
|---|---|---|---|---|---|---|---|
| 1 | Saudi Arabia (OPEC) | 8,336 | 1,325 | 7,322 | 1,164 | 8,651 | 1,376 |
| 2 | Russia 1 | 7,083 | 1,126 | 7,194 | 1,144 | 6,565 | 1,044 |
| 3 | Iran (OPEC) | 2,540 | 403 | 2,486 | 395 | 2,519 | 401 |
| 4 | United Arab Emirates (OPEC) | 2,524 | 401 | 2,303 | 366 | 2,515 | 400 |
| 5 | Kuwait (OPEC) | 2,343 | 373 | 2,124 | 338 | 2,150 | 342 |
| 6 | Nigeria (OPEC) | 2,257 | 359 | 1,939 | 308 | 2,146 | 341 |
| 7 | Iraq (OPEC) | 1,915 | 304 | 1,764 | 280 | 1,438 | 229 |
| 8 | Angola (OPEC) | 1,760 | 280 | 1,878 | 299 | 1,363 | 217 |
| 9 | Norway 1 | 1,752 | 279 | 2,132 | 339 | 2,542 | 404 |
| 10 | Venezuela (OPEC) 1 | 1,715 | 273 | 1,748 | 278 | 2,203 | 350 |
| 11 | Algeria (OPEC) 1 | 1,568 | 249 | 1,767 | 281 | 1,847 | 297 |
| 12 | Qatar (OPEC) | 1,468 | 233 | 1,066 | 169 | – | – |
| 13 | Canada 2 | 1,405 | 223 | 1,168 | 187 | 1,071 | 170 |
| 14 | Kazakhstan | 1,396 | 222 | 1,299 | 207 | 1,114 | 177 |
| 15 | Azerbaijan 1 | 836 | 133 | 912 | 145 | 532 | 85 |
| 16 | Trinidad and Tobago 1 | 177 | 112 | 167 | 160 | 155 | 199 |
1 peak production already passed in this state
2 Canadian statistics are complicated by the fact it is both an importer and exporter of crude oil, and refines large amounts of oil for the U.S. market. It is the leading source of U.S. imports of oil and products, averaging 2,500,000 bbl/d (400,000 m3/d) in August 2007.[83]
Total world production/consumption (as of 2005) is approximately 84 million barrels per day (13,400,000 m3/d).
Import
Oil imports by country (barrels per day, 2006)
| # | Importing nation | 103bbl/day (2011) | 103m3/day (2011) | 103bbl/day (2009) | 103m3/day (2009) | 103bbl/day (2006) | 103m3/day (2006) |
|---|---|---|---|---|---|---|---|
| 1 | United States 1 | 8,728 | 1,388 | 9,631 | 1,531 | 12,220 | 1,943 |
| 2 | China 2 | 5,487 | 872 | 4,328 | 688 | 3,438 | 547 |
| 3 | Japan | 4,329 | 688 | 4,235 | 673 | 5,097 | 810 |
| 4 | India | 2,349 | 373 | 2,233 | 355 | 1,687 | 268 |
| 5 | Germany | 2,235 | 355 | 2,323 | 369 | 2,483 | 395 |
| 6 | South Korea | 2,170 | 345 | 2,139 | 340 | 2,150 | 342 |
| 7 | France | 1,697 | 270 | 1,749 | 278 | 1,893 | 301 |
| 8 | Spain | 1,346 | 214 | 1,439 | 229 | 1,555 | 247 |
| 9 | Italy | 1,292 | 205 | 1,381 | 220 | 1,558 | 248 |
| 10 | Singapore | 1,172 | 186 | 916 | 146 | 787 | 125 |
| 11 | Republic of China (Taiwan) | 1,009 | 160 | 944 | 150 | 942 | 150 |
| 12 | Netherlands | 948 | 151 | 973 | 155 | 936 | 149 |
| 13 | Turkey | 650 | 103 | 650 | 103 | 576 | 92 |
| 14 | Belgium | 634 | 101 | 597 | 95 | 546 | 87 |
| 15 | Thailand | 592 | 94 | 538 | 86 | 606 | 96 |
1 peak production of oil expected in 2020[84]
2 Major oil producer whose production is still increasing[citation needed]
Oil Imports to the USA by country 2010
Oil imports to US, 2010
Non-producing consumers
Countries whose oil production is 10% or less of their consumption.| # | Consuming nation | (bbl/day) | (m³/day) |
|---|---|---|---|
| 1 | Japan | 5,578,000 | 886,831 |
| 2 | Germany | 2,677,000 | 425,609 |
| 3 | South Korea | 2,061,000 | 327,673 |
| 4 | France | 2,060,000 | 327,514 |
| 5 | Italy | 1,874,000 | 297,942 |
| 6 | Spain | 1,537,000 | 244,363 |
| 7 | Netherlands | 946,700 | 150,513 |
| 8 | Turkey | 575,011 | 91,663 |
Environmental effects
Diesel fuel spill on a road
Ocean acidification
Seawater acidification
Global warming
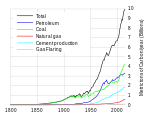
When burned, petroleum releases carbon dioxide, a greenhouse gas. Along with the burning of coal, petroleum combustion may be the largest contributor to the increase in atmospheric CO2.[citation needed] Atmospheric CO2 has risen over the last 150 years to current levels of over 390 ppmv, from the 180 – 300 ppmv of the prior 800 thousand years[87][88][89] This rise in temperature may have reduced the Arctic ice cap to 1,100,000 sq mi (2,800,000 km2),[citation needed] smaller than ever recorded.[90] Because of this melt, more oil reserves have been revealed. It is estimated by the International Energy Agency that about 13 percent of the world's undiscovered oil resides in the Arctic.[91]Extraction
Oil extraction is simply the removal of oil from the reservoir (oil pool). Oil is often recovered as a water-in-oil emulsion, and specialty chemicals called demulsifiers are used to separate the oil from water. Oil extraction is costly and sometimes environmentally damaging. Offshore exploration and extraction of oil disturbs the surrounding marine environment.[92]Oil spills
Oil slick from the Montara oil spill in the Timor Sea, September, 2009
Volunteers cleaning up the aftermath of the Prestige oil spill
The quantity of oil spilled during accidents has ranged from a few hundred tons to several hundred thousand tons (e.g., Deepwater Horizon oil spill, SS Atlantic Empress, Amoco Cadiz). Smaller spills have already proven to have a great impact on ecosystems, such as the Exxon Valdez oil spill.
Oil spills at sea are generally much more damaging than those on land, since they can spread for hundreds of nautical miles in a thin oil slick which can cover beaches with a thin coating of oil. This can kill sea birds, mammals, shellfish and other organisms it coats. Oil spills on land are more readily containable if a makeshift earth dam can be rapidly bulldozed around the spill site before most of the oil escapes, and land animals can avoid the oil more easily.
Control of oil spills is difficult, requires ad hoc methods, and often a large amount of manpower. The dropping of bombs and incendiary devices from aircraft on the SS Torrey Canyon wreck produced poor results;[93] modern techniques would include pumping the oil from the wreck, like in the Prestige oil spill or the Erika oil spill.[94]
Though crude oil is predominantly composed of various hydrocarbons, certain nitrogen heterocylic compounds, such as pyridine, picoline, and quinoline are reported as contaminants associated with crude oil, as well as facilities processing oil shale or coal, and have also been found at legacy wood treatment sites. These compounds have a very high water solubility, and thus tend to dissolve and move with water. Certain naturally occurring bacteria, such as Micrococcus, Arthrobacter, and Rhodococcus have been shown to degrade these contaminants.[95]
Tarballs
A tarball is a blob of crude oil (not to be confused with tar, which is a man-made product derived from pine trees or refined from petroleum) which has been weathered after floating in the ocean. Tarballs are an aquatic pollutant in most environments, although they can occur naturally, for example in the Santa Barbara Channel of California[96][97] or in the Gulf of Mexico off Texas.[98] Their concentration and features have been used to assess the extent of oil spills. Their composition can be used to identify their sources of origin,[99][100] and tarballs themselves may be dispersed over long distances by deep sea currents.[97] They are slowly decomposed by bacteria, including Chromobacterium violaceum, Cladosporium resinae, Bacillus submarinus, Micrococcus varians, Pseudomonas aeruginosa, Candida marina and Saccharomyces estuari.[96]Whales
James S. Robbins has argued that the advent of petroleum-refined kerosene saved some species of great whales from extinction by providing an inexpensive substitute for whale oil, thus eliminating the economic imperative for open-boat whaling.[101]Alternatives to petroleum
In the United States in 2007 about 70 percent of petroleum was used for transportation (e.g. gasoline, diesel, jet fuel), 24 percent by industry (e.g. production of plastics), 5 percent for residential and commercial uses, and 2 percent for electricity production.[102] Outside of the US, a higher proportion of petroleum tends to be used for electricity.[103]Alternatives to petroleum-based vehicle fuels
Brazilian fuel station with four alternative fuels for sale: diesel (B3), gasohol (E25), neat ethanol (E100), and compressed natural gas (CNG)
- Vehicles that use alternative fuels used in standard or modified internal combustion engines such as natural gas vehicles, neat ethanol vehicles, flexible-fuel vehicles, biodiesel-powered vehicles, propane autogas, and hydrogen vehicles.
- Vehicles with advanced propulsion systems that reduce or substitute petroleum use such as battery electric vehicles, plug-in hybrid electric vehicles, hybrid electric vehicles, and hydrogen fuel cell vehicles.
Alternatives to using oil in industry
Biological feedstocks do exist for industrial uses such as Bioplastic production.[104]Alternatives to burning petroleum for electricity
In oil producing countries with little refinery capacity, oil is sometimes burned to produce electricity. Renewable energy technologies such as solar power, wind power, micro hydro, biomass and biofuels are used, but the primary alternatives remain large-scale hydroelectricity, nuclear and coal-fired generation.Future of petroleum production
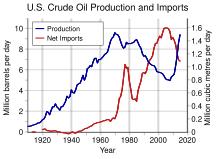
US oil production and imports, 1910-2012
In 2016 Goldman Sachs predicted lower demand for oil due to emerging economies concerns, especially China.[105] The BRICS (Brasil, Russia, India, China, South Africa) countries might also kick in, as China briefly was the first automobile market in December 2009.[106] The immediate outlook still hints upwards. In the long term, uncertainties linger; the OPEC believes that the OECD countries will push low consumption policies at some point in the future; when that happens, it will definitely curb oil sales, and both OPEC and the Energy Information Administration (EIA) kept lowering their 2020 consumption estimates during the past five years.[107] Oil products increasingly compete with alternative sources, mainly coal and natural gas, both cheaper sources. Production will also face an increasingly complex situation; while OPEC countries still have large reserves at low production prices, newly found reservoirs often lead to higher prices; offshore giants such as Tupi, Guara and Tiber demand high investments and ever-increasing technological abilities. Subsalt reservoirs such as Tupi were unknown in the twentieth century, mainly because the industry was unable to probe them. Enhanced Oil Recovery (EOR) techniques (example: DaQing, China[108] ) will continue to play a major role in increasing the world's recoverable oil.
The expected of available petroleum resources has always been around 35 years or even less since the start of the modern exploration. The oil constant, a insider pun in the German industry refers to that effect.[109]
Peak oil
Global peak oil forecast
Hubbert applied his theory to accurately predict the peak of U.S. conventional oil production at a date between 1966 and 1970. This prediction was based on data available at the time of his publication in 1956. In the same paper, Hubbert predicts world peak oil in "half a century" after his publication, which would be 2006.[111]
It is difficult to predict the oil peak in any given region, due to the lack of knowledge and/or transparency in accounting of global oil reserves.[112] Based on available production data, proponents have previously predicted the peak for the world to be in years 1989, 1995, or 1995–2000. Some of these predictions date from before the recession of the early 1980s, and the consequent reduction in global consumption, the effect of which was to delay the date of any peak by several years. Just as the 1971 U.S. peak in oil production was only clearly recognized after the fact, a peak in world production will be difficult to discern until production clearly drops off.[113] The peak is also a moving target as it is now measured as "liquids", which includes synthetic fuels, instead of just conventional oil.[114]
The International Energy Agency (IEA) said in 2010 that production of conventional crude oil had peaked in 2006 at 70 MBBL/d, then flattened at 68 or 69 thereafter.[115][116] Since virtually all economic sectors rely heavily on petroleum, peak oil, if it were to occur, could lead to a "partial or complete failure of markets".[117] In the mid-2000s, widespread fears of an imminent peak led to the "peak oil movement," in which over one hundred thousand Americans prepared, individually and collectively, for the "post-carbon" future.[118]
Unconventional production
The calculus for peak oil has changed with the introduction of unconventional production methods. In particular, the combination of horizontal drilling and hydraulic fracturing has resulted in a significant increase in production from previously uneconomic plays.[119] Analysts expect that $150 billion will be spent on further developing North American tight oil fields in 2015. The large increase in tight oil production is one of the reasons behind the price drop in late 2014.[120] Certain rock strata contain hydrocarbons but have low permeability and are not thick from a vertical perspective. Conventional vertical wells would be unable to economically retrieve these hydrocarbons. Horizontal drilling, extending horizontally through the strata, permits the well to access a much greater volume of the strata. Hydraulic fracturing creates greater permeability and increases hydrocarbon flow to the wellbore.See also
Notes
|url= value (help) (PDF). Reviews of Geophysics. 27 (1): 115–139. Bibcode:1989RvGeo..27..115M. doi:10.1029/RG027i001p00115.U.S. oil production has grown rapidly in recent years. U.S. Energy Information Administration (EIA) data, which reflect combined production of crude oil and lease condensate, show a rise from 5.7 million barrels per day (bbl/d) in 2011 to 7.4 million bbl/d in 2013. EIA's Short-Term Energy Outlook (STEO) projects continuing rapid production growth in 2014 and 2015, with forecast production in 2015 reaching 9.2 million bbl/d. Beyond 2015, EIA's Annual Energy Outlook (AEO) projects further production growth, although its pace and duration remain uncertain. Domestic production plateaus near 9.6 million bbl/d between 2017 and 2020, close to its historic high of 9.6 million bbl/d in 1970, in the AEO2014 Reference case. In the AEO2014 High Oil and Gas Resource case, growth continues through the 2020s and into the 2030s, with production reaching 13.3 million barrels per day in 2036.
- Ovale, Peder. "Her ser du hvorfor oljeprisen faller" In English Teknisk Ukeblad, 11 December 2014. Accessed: 11 December 2014.
References
- Akiner, Shirin; Aldis, Anne, eds. (2004). The Caspian: Politics, Energy and Security. New York: Routledge. ISBN 978-0-7007-0501-6.
- Bauer Georg, Bandy Mark Chance (tr.), Bandy Jean A.(tr.) (1546). De Natura Fossilium. vi (in Latin). translated 1955
- Hyne, Norman J. (2001). Nontechnical Guide to Petroleum Geology, Exploration, Drilling, and Production. PennWell Corporation. ISBN 0-87814-823-X.
- Mabro, Robert; Organization of Petroleum Exporting Countries (2006). Oil in the 21st century: issues, challenges and opportunities. Oxford Press. ISBN 978-0-19-920738-1.
- Maugeri, Leonardo (2005). The Age of Oil: What They Don't Want You to Know About the World's Most Controversial Resource. Guilford, CT: Globe Pequot. p. 15. ISBN 978-1-59921-118-3.
- Speight, James G. (1999). The Chemistry and Technology of Petroleum. Marcel Dekker. ISBN 0-8247-0217-4.
- Speight, James G; Ancheyta, Jorge, eds. (2007). Hydroprocessing of Heavy Oils and Residua. CRC Press. ISBN 0-8493-7419-7.
- Vassiliou, Marius (2009). Historical Dictionary of the Petroleum Industry. Scarecrow Press (Rowman & Littlefield). ISBN 0-8108-5993-9.
Further reading
- Kenney, J., Kutcherov, V., Bendeliani, N. and Alekseev, V. (2002). "The
evolution of multicomponent systems at high pressures: VI. The
thermodynamic stability of the hydrogen–carbon system: The genesis of
hydrocarbons and the origin of petroleum". Proceedings of the National Academy of Sciences of the United States of America. 99 (17): 10976–10981. arXiv:physics/0505003
 . Bibcode:2002PNAS...9910976K. doi:10.1073/pnas.172376899. PMC 123195
. Bibcode:2002PNAS...9910976K. doi:10.1073/pnas.172376899. PMC 123195  . PMID 12177438.
. PMID 12177438. - Khavari, Farid A. (1990). Oil and Islam: the Ticking Bomb. First ed. Malibu, Calif.: Roundtable Publications. viii, 277 p., ill. with maps and charts. ISBN 0-915677-55-5
- GA Mansoori, N Enayati, LB Agyarko (2016), Energy: Sources, Utilization, Legislation, Sustainability, Illinois as Model State, World Sci. Pub. Co., ISBN 978-981-4704-00-7
External links
| Wikimedia Commons has media related to Petroleum. |
| Wikisource has the text of the 1905 New International Encyclopedia article Petroleum. |
- American Petroleum Institute – the trade association of the US oil industry.
- U.S. Energy Information Administration
- Information on Petroleum and Crude Oil
- Petroleum Online e-Learning resource from IHRDC
- Petroleum Community Forum
- Geo ExPro (Petroleum Geoscience Magazine)
- Joint Organisations Data Initiative | Oil and Gas Data Transparency
- U.S. National Library of Medicine: Hazardous Substances Databank – Crude Oil
- Natural petroleum - intensification distillation, increase volume of light fractions
- Crude: 2007 Australian Broadcasting Corporation documentary about the formation








![K={\frac {1.62}{API}}[1-0.0003(t-32)]](https://wikimedia.org/api/rest_v1/media/math/render/svg/6241f010aae8f8eff354761338cdef95bc22922e)


















Comments
Post a Comment